Triana-González Salma1, Cano-Díaz Ana Luz1, Mata-Marín José Antonio1, Gaytán-Martínez Jesús Enrique1
- Infectious Diseases Department, Hospital de Infectología “Dr. Daniel Méndez Hernández” La Raza National Medical
Center, Instituto Mexicano del Seguro Social, Mexico City, Mexico.
Corresponding author: José Antonio Mata Marín. Hospital de Infectología “Dr. Daniel Méndez Hernández” La Raza National Medical Center, Instituto Mexicano del Seguro Social. Seris y Vallejo S/N. Colonia La Raza. Zip code: 02990. Delegación Azcapotzalco, Mexico City, Mexico. Phone: (55)57245900 Ext. 23924. E-mail: jamatamarin@gmail.com
ABSTRACT
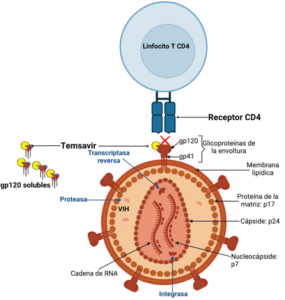
In accordance with recommendations from other countries, the Mexican guidelines positions integrase strand transfer inhibitors (INSTIs) as the preferred third component of combined antiretroviral therapy (ART), restricting protea se inhibitors (PI) and non-nucleoside reverse transcriptase inhibitors (NNRTIs) to specific indications (4); in addition, the use of NNRTIs is often limited by drug resistance pro files and neuropsychiatric adverse events associated with
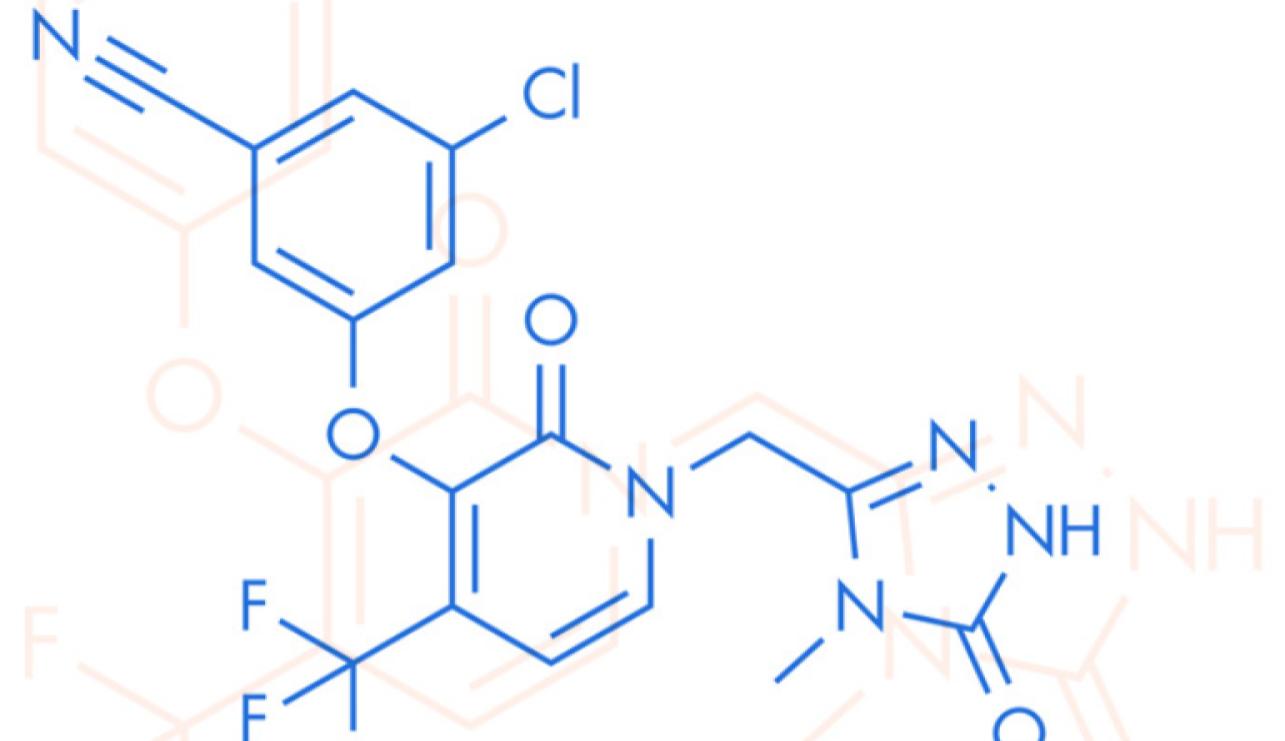
first-generation drugs (5). In consequence, development of doravirine (DOR), a second generation NNRTI, focused on improving tolerability and stability against common mutations with promising results derived from randomized clinical trials (6). The greatest amount of data existing on DOR is derived from the DRIVE trials that assessed treatment-naïve patients as well as those switching from stable ART (DRI-VE-FORWARD, DRIVE-AHEAD, DRIVE-SHIFT) (7, 8, 9). These trials showed a consistent trend of DOR being not inferior in terms of virological outcomes and safety profile, leading to approval by the US Food and Drug Administration (10); however, effectiveness and safety data under real world conditions is still lacking, moreover, patient population deriving the most benefit from this regimen remains to against commonbe defined. (7). The aim of this study was to assess indications for active switching to a DOR-based regimen and to characterize effectiveness and differences on body weight and neuropsychiatric scales (NPS) at baseline and at 24 weeks of follow under real world conditions.
MATERIAL AND METHODS
Design
A single-center case series study was conducted from April 2022 to July 2023 in patients treated in HIV Clinic at Hospital de Infectología “La Raza” National Medical Center, Mexico City. This center is a third level reference unit for the management of HIV and other infectious diseases in people with social security coverage. Information was obtained retrospectively at baseline and at 24 weeks of follow. The primary objective was to describe the proportion of patients with virological suppression at 24 weeks and potential differences on body weight and NPS after regimen switch. Written informed consent was not a requirement given the descriptive and retrospective nature of the study.
Study population
Study subjects were ≥18 years old PWH stable on ARV and virologically suppress that were switched to a DOR/3TC/ TDF regimen and that were followed for at least 24 weeks after switch.
Measurements
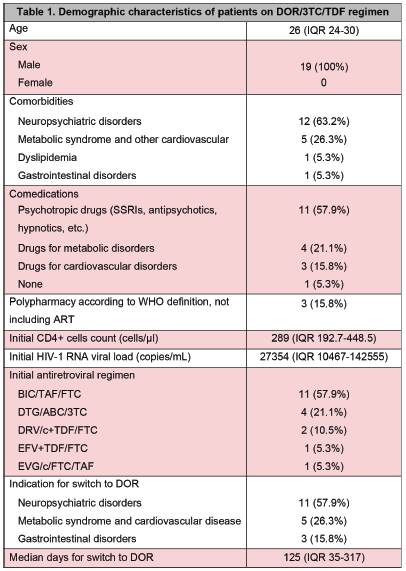
Demographic and clinical data was collected from each consult visit at the HIV Clinic and included a description of comorbidities and comedications that were classified according to type of disease and drug. The medical indication for switch was classified according to comorbidities. Laboratory data (CD4+ cells count and HIV-1 RNA viral load) was also evaluated including baseline measures and at 24 weeks of switch. Body weight measure was standardized with Beurer BF105 scale. Self-questionnaire neuropsychiatric scales included: Hospital Anxiety and Depression Scale (HADS), Insomnia Severity Index (ISI) and Patient Health Questionnaire (PHQ-9), all of which were previously validated in PWH. (Table 1).
Statistical analysis
Qualitative data was analyzed through frequency and percentage tables. Quantitative data, according to their non-normal distribution (Kolmogorov-Smirnov test), was summarized using medians and interquartile ranges (IQR). Exploratory analysis included comparisons using Wilcoxon test. A p value ≤0.05 with a confidence interval (CI) of 95% was considered statistically significant. All analyses were conducted using SPSS software (version 26; IBM® SPSS® Corp., Armonk, NJ, USA).
RESULTS
wenty-one PWH were initially eligible based on their ART (DOR/3TC/TDF regimen) and 19 were included in this case series. The total of subjects were men, and the median age was 26 years old (95%CI, IQR 24-30). Median CD4+ cells count and HIV-1 RNA viral load at diagnosis was 289 cells/ μl (95%CI, IQR 192-448) and 27354 copies/mL (95%CI, IQR 10467-142555), respectively. Most patients were on an INSTI-based regimen before switch to DOR/3TC/TDF regimen and BIC/TAF/FTC 11 (57.9%) was the most prescribed regimen. One (5.3%) subject was switched from a first generation NNRTI and 1 (5.3%) was switched from PI.
Most frequent reported comorbidities were neuropsychiatric disorders 12 (63.2%), followed by metabolic and cardiovascular diseases 5 (26.3%). Psychotropic drugs were prescribed in addition to ART in 11 (57.9%) subjects and 3 (15.8%) fulfilled polypharmacy definition. Main indication for switch to a DOR/3TC/TDF regimen was to improve tolerability of ART due to neuropsychiatric symptoms in 11 (57.9%) cases. The median of days for switching from first
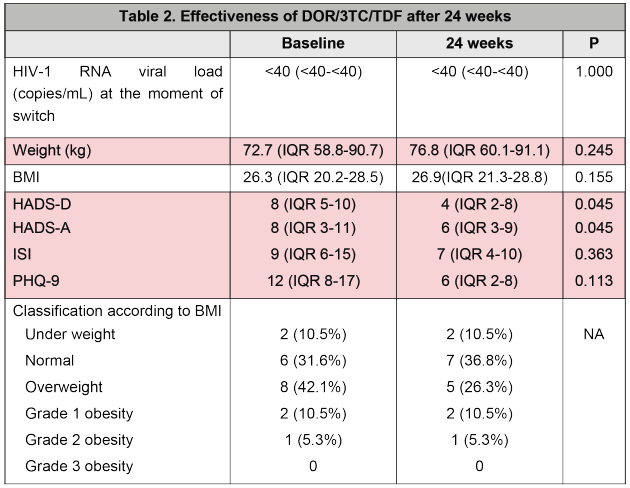
regimen to a DOR/3TC/TDF regimen was 125 (95%CI, IQR 35-317) (Table 1). Median BMI before and after switch was 26.3 (95%CI, IQR 20.2-28.5) and 26.9 (95%CI, IQR 21.3-28.8), respectively (p=0.155). On the other hand, favorable changes were documented after the switch in HADS-D and HADS-A at baseline and at 24 weeks, 8 (95% CI, IQR 5-10) vs. 4 (95% CI, IQR 2-8) (p=0.045) and 8 (95% CI, IQR 3-11) vs. 6 (95% CI, IQR 3-9) (p=0.045), respectively. No cases of virological failure were reported, with undetectable viral load (<40 copies/mL) at 24 weeks of switching in all patients switched (Table 2).
DISCUSSION
In this case series, we found that patient deriving the most benefit from switch to a DOR/3TC/TDF regimen are PWH who have neuropsychiatric and metabolic comorbidities, in addition to some contraindication for the use of preferred regimens in local guidelines. Most patients included in analysis were previously treated with an INSTI and they were switched due to exacerbation of neuropsychiatric symptoms or metabolic alterations associated with initial treatment. In this report, DOR was effective in maintaining virological suppression and also showed a favorable profile regarding weight gain and neuropsychiatric scales. Subjects in this study are younger than previously reported by O’Halloran et al., who found that 45% of patients with a DOR-based regimen were older than 50 years old, however in that cohort, authors also described a high frequency of comorbidities, most commonly depression, similar to findings in our population (11). Regarding virological effectiveness, Lanting et al., found that virologic failure occurred in less than 1% of patients with DOR-based therapy and this is concordant to data after 24 weeks in this case series (12). Exploratory analysis in this study found a favorable profile regarding weight gain and neuropsychiatric scales after switch to a second-generation NNRTI, as previously reported in clinical trials (7, 8, 9).
This study has some limitations. In first place the small number of participants limits generalization of findings; however, doravirine prescription is currently limited to few indications. In second place, only men were included. Finally, we limited metabolic outcomes to changes in body weight, but it would be necessary complete with body composition. Despite limitations, a key strength of this study is that, to our knowledge, this is the first report of DOR use under real world conditions in Latin American. Moreover, although information was obtained in 19 men, it was based on objective data (validated neuropsychiatric scales) which made it possible to quantify a potential favorable effect of regimen in patients with neuropsychiatric comorbidities. More studies with a bigger sample size and more metabolic 4 weeks of switching in 19/19 measurements are necessary to better define PWH with the most benefit from switch to a DOR-based regimen.
In conclusion, real-world data from this study suggest that DOR is effective in maintaining viral suppression with a potentially favorable profile in NPS.
REFERENCES
- Menéndez-Arias L, Delgado R. Update and latest advances in antiretroviral therapy. Trends Pharmacol Sci. 2022;43(1):16-29. doi:10.1016/j.tips.2021.10.004.
- Ambrosioni J, Levi L, Alagaratnam J, et al. Major revision version 12.0 of the European AIDS Clinical Society guidelines 2023. HIV Med. 2023;24(11):1126-1136. doi:10.1111/hiv.13542.
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. 2023. Available at https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv.
- Guía de manejo antirretroviral de las personas con VIH. México: Censida/Secretaría de Salud. Available at https://www.gob.mx/censida/documentos/guia-de-manejo-antirretroviral-de-las-personas-con-vih-mexico-2021-297710
- Vanangamudi M, Kurup S, Namasivayam V. Non-nu-cleoside reverse transcriptase inhibitors (NNRTIs): a brief overview of clinically approved drugs and combination regimens. Curr Opin Pharmacol. 2020;54:179 doi:10.1016/j.coph.2020.10.009.
- Stockdale AJ, Khoo S. Doravirine: its role in HIV treatment. Curr Opin HIV AIDS. 2022;17(1):4-14. doi:10.1097/COH.0000000000000709
- Molina JM, Squires K, Sax PE, et al. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVE-FORWARD): 96-week results of a randomised, double-blind, non-inferiority, phase 3 trial. Lancet HIV. 2020;7(1):e16-e26. doi:10.1016/S2352-3018(19)30336-4
- Orkin C, Squires KE, Molina JM, et al. Doravirine/Lamivudine/Tenofovir Disoproxil Fumarate (TDF) Versus Efavirenz/Emtricitabine/TDF in Treatment-naive Adults With Human Immunodeficiency Virus Type 1 Infection: Week 96 Results of the Randomized, Dou-ble-blind, Phase 3 DRIVE-AHEAD Noninferiority Trial. Clin Infect Dis. 2021;73(1):33-42. doi:10.1093/cid/ciaa822.
- Johnson M, Kumar P, Molina JM, et al. Switching to Doravirine/Lamivudine/Tenofovir Disoproxil Fumarate (DOR/3TC/TDF) Maintains HIV-1 Virologic Suppression Through 48 Weeks: Results of the DRIVE-SHIFT Trial. J Acquir Immune Defic Syndr. 2019;81(4):463-doi:10.1097/QAI.0000000000002056
- Rock AE, Lerner J, Badowski ME. Doravirine and Its Potential in the Treatment of HIV: An Evidence-Based Review of the Emerging Data. HIV AIDS (Auckl). 2020;12:201-210. Published 2020 Jun 10. doi:10.2147/HIV.S184018
- O’Halloran C, Gilleece Y, Leung S, et al. Real world utilisation of doravirine among people living with human immunodeficiency virus in England (DRIVE-REAL). Int J STD AIDS. doi:10.1177/09564624231215977.
- Lanting VR, Oosterhof P, Moha DA, et al. Switching to doravirine in cART experienced patients: An effective and highly tolerated option with substantial cost savings. J Acquir Immune Defic Syndr. doi:10.1097/QAI.0000000000003337.