Rodríguez Evaristo Mara Soraya1, Mata Marin José Antonio2, Salinas Velázquez Gloria Elizabeth3, Méndez Gaytán, Juan Francisco3, Cortés Vargas Fabiola4, Pompa-Mera Ericka5, Meneses Cisneros Betzahida6, Pérez-Barragán Edgar2, Núñez-Armendaris Mireya2, Barragán-Huerta Laura7, Gaytan Martinez Jesús Enrique2 (SIDVI-Study group).
- Internal Medicine Department, General Hospital, “La Raza” National Medical Center, Instituto Mexicano del Seguro
Social, Mexico City, México.
- Infectious Diseases Department, Hospital de Infectología “La Raza” National Medical Center, Instituto Mexicano del
Seguro Social, Mexico City, México. - Imagenology Department, Hospital de Infectología “La Raza” National Medical Center, Instituto Mexicano del Seguro Social, Mexico City, México.
- Nutrional Department, Hospital de Infectología “La Raza” National Medical Center, Instituto Mexicano del Seguro
Social, Mexico City, México. - Research Unit, Hospital de Infectología “La Raza” National Medical Center, Instituto Mexicano del Seguro Social,
Mexico City, México. - Electrocardiography Department, Hospital de Infectología “La Raza” National Medical Center, Instituto Mexicano del
Seguro Social, Mexico City, México. - Biological Science and Health Department, Universidad Autónoma Metropolitana, Campus Xochimilco, México City,
México
Correspondence author:
José Antonio Mata Marín (jamatamarin@gmail.com)
Address: Hospital de Infectología “La Raza” National Medical Center. Seris y Vallejo S/N. Colonia La Raza. Postal code: 02990. Delegación Azcapotzalco, Mexico. Phone:
(55)57245900 ext 23924, jamatamarin@sidvi
Abstract
Objective: Recent evidence suggests that patients starting an INSTI based regimen are more likely to experience weight gain and metabolic syndrome (MetS) than those on antiretroviral regimen based on other classes. The aim of the study was to identify the incidence of MetS at 24 weeks for ATP III score in patients living with HIV (PLHIV) who started antiretroviral therapy (ART) with Dolutegravir based-regimen compared with Bictegravir based-regimen.
Methods: a randomized open clinical trial was developed with men PLHIV who started ART randomized to BIC/FTC/TAF or DTG/3TC/ABC. Weight, height, blood pressure and waist circumference were measured during routine clinical care; in addition, bioimpedance was done; repeated measures of weight in kilograms were done at baseline and 24 weeks. Metabolic laboratory tests were done at the same time.
Results: From 278 patients invited to participate, 176 accepted to participate and they were randomized to BIC/TAF/FTC or DTG/ABC/3TC. Of these, 78 reached 24 weeks of treatment for the analysis. At 24 weeks, an incidence of 2 (5.7%) and 3 (6.9%) cases of metabolic syndrome was reported in BIC/ TAF/FTC and DTG/ABC/3TC groups respectively. No significant difference was found between the incidence of metabolic syndrome, incidence of hypertriglyceridemia and weight gain when both groups were compared.
Conclusion: This Mexican trial shows that the incidence of MetS was similar in both groups. Second generation INSTI-based regimens were effective and well tolerated in our population.
Resumen
Objetivo: hay evidencia que sugiere que los pacientes que inician un régimen con INSTI es más probable que experimenten ganancia de peso y síndrome metabólico (SMet) que los que
usan un régimen con otras clases de antirretrovirales. El objetivo de este estudio fue identificar la incidencia de SMet a 24 semanas por ATP III en personas que viven con VIH (PVVIH) que inician terapia antirretroviral (TAR) con un régimen con Dolutegravir comparado con un régimen con Bictegravir.
Métodos: se realizó un ensayo clínico aleatorizado abierto con pacientes hombres que viven con VIH (PVVIH) que iniciaron un tratamiento aleatorizados a BIC/FTC/TAF o DTG/3TC/ ABC. Se midió peso, talla, presión arterial y circunferencia abdominal durante la evaluación rutinaria; además, se efectuó bioimpedancia y se repitió a 24 semanas así como pruebas de laboratorio metabólicas.
Resultados: de 278 pacientes invitados a participar, 176 aceptaron y fueron aleatorizados a BIC/TAF/FTC o DTG/ABC/3TC. De ellos, 78 alcanzaron 24 semanas de tratamiento para este análisis. A la semana 24, se tuvo una incidencia de 2 (5.7%) y 3 (6.9%) casos de síndrome metabólico en el grupo de BIC/TAF/FTC y DTG/ABC/3TC respectivamente. No se encontró diferencia significativa en la incidencia de síndrome metabólico, hipertrigliceridemia y ganancia de peso en ambos grupos.
Conclusión: este ensayo clínico Mexicano muestra que la incidencia de SMet fue similar en ambos grupos. Un régimen con INSTI de segunda generación fue efectivo y bien tolerado en nuestra población.
Background
Integrase strand transferase inhibitor (INSTI) class has demonstrated a good tolerability profile. However, recent evidence suggests that patients starting an INSTI based regimen are more likely to experience weight gain than those on antiretroviral regimen based on other classes (1, 2).
Tenofovir Alafenamide (TAF) has been associated with increased weight in patients living with HIV (PLHIV) (3). INSTI use has been associated with higher body mass index (BMI) and waist circumference (WC) and greater odds of obesity but inconsistently associated with metabolic syndrome (MetS) (4). However, these possible causes for weight gain have to be balanced against an increase in obesity due to diet and sedentary lifestyle in the general population also affecting PLHIV (5).
Cardio-metabolic abnormalities frequently cluster and manifest as the MetS, a constellation of interrelated metabolic disorders comprising abdominal obesity, raised blood pressure, dyslipidemia and hyperglycemia. The importance of the MetS is that it is a powerful predictor of future cardiovascular disease and type 2 diabetes (T2DM) (7,8).
The aim of this study was to compare the incidence of MetS at 24 weeks for ATP III score in patients living with HIV who started antiretroviral therapy (ART) with Dolutegravir based-regimen vs. Bictegravir based-regimen.
Methods
Design
An open-label randomized clinical trial was conducted, the primary endpoint was the incidence of MetS at 144 weeks comparing BIC/FTC/TAF with DTG/3TC/ABC in patients with HIV type 1 (HIV-1) infection starting ART; here we present analysis at 24 weeks of treatment with safety data.
The study was approved by the local committee for health research 3502 at the Hospital de Infectología “La Raza” National Medical Center, with registration number R-2021-3502-084. Written informed consent was obtained from all participants.
Study population
Study subjects were men who have sex with men (MSM) with HIV infection ≥18 years old naïve to ART (PLHIV). The trial enrolled residents of Mexico City and Mexico State from February 2021 through December 2022. Inclusion criteria were an age of 18 years or older, a viral load of 500 copies or more per milliliter, and a creatinine clearance of more than 60 ml per minute (CKD-EPI formula). Among the exclusion criteria were MetS, comorbidities or prescription of drugs associated with weight gain or weight loss or current treatment for tuberculosis. Each patient received a personalized eating plan based on the equivalent food system method adjusted to their energy requirements before starting treatment.
Measurements
The primary endpoint was the incidence of MetS at week 24. Secondary objectives were the percentage of patients with an HIV-1 RNA level of less than 40 copies per milliliter, CD4+
count changes, and side-effect profile and safety, including findings on physical examination and laboratory analyses. Body composition was measured baseline and at 24 weeks using a
next generation bioimpedance device. Each patient was measured in duplicate to ensure better results and to avoid minimal changes. An ultrasonography to measure visceral and subcutaneous fat was measured. Waist circumference was also measured in the fasting state by using a non-retractable material flexible ruler.
There was no testing of HIV resistance at screening. Patients with two confirmed elevations in the HIV-1 RNA level to 1000 copies or more per milliliter after week 24 were tested for drug resistance, together with their stored baseline samples.
Weight and height were measured during routine clinical care, and recorded in electronic health records. Repeated measures of weight in kilograms (kg) were done at baseline and at 24 weeks.
The laboratory tests performed were glucose, triglycerides (TG), total cholesterol (TC), HDL-cholesterol (HDL-c), and LDL-cholesterol (LDL-c). Metabolic disturbances were defined as impaired fasting glucose (≥100 mg/dL), hypertriglyceridemia (≥150 mg/dL), low HDL-cholesterol (≤40 mg/dL). In addition, clinical data were collected as follows: weight, height, arterial pressure and history of comorbidities. We calculated BMI, and it was classified as 18.5 to <25 kg/m2 healthy weight, 25.0 to <30 kg/m2 overweight and 30.0 kg/m2 or higher obesity.
Statistical analysis
Descriptive results were summarized using median and interquartile range (IQR). Percentage was obtained in order to evaluate the weight gain classification. The main outcome variable was the incidence of MS at 24 weeks. We assessed weight change and metabolic changes at 24 weeks with a Wilcoxon signed-rank test. Mann Whitney U-test was used to compare between groups. The effectiveness outcomes were analyzed in the intention-to-treat population and the safety outcomes were analyzed in the as-treated population. All analyses were conducted using SPSS software (version 22; SPSS IBM Corp., Armonk, NJ, USA)
Results
From 278 patients invited to participate, 176 accepted to participate and they were randomized to BIC/TAF/FTC or DTG/ABC/3TC. Of these, 78 reached 24 weeks of treatment for the analysis. Fifteen participants withdrew from the study due to adverse events and eight due to loss of social security (Figure 1).
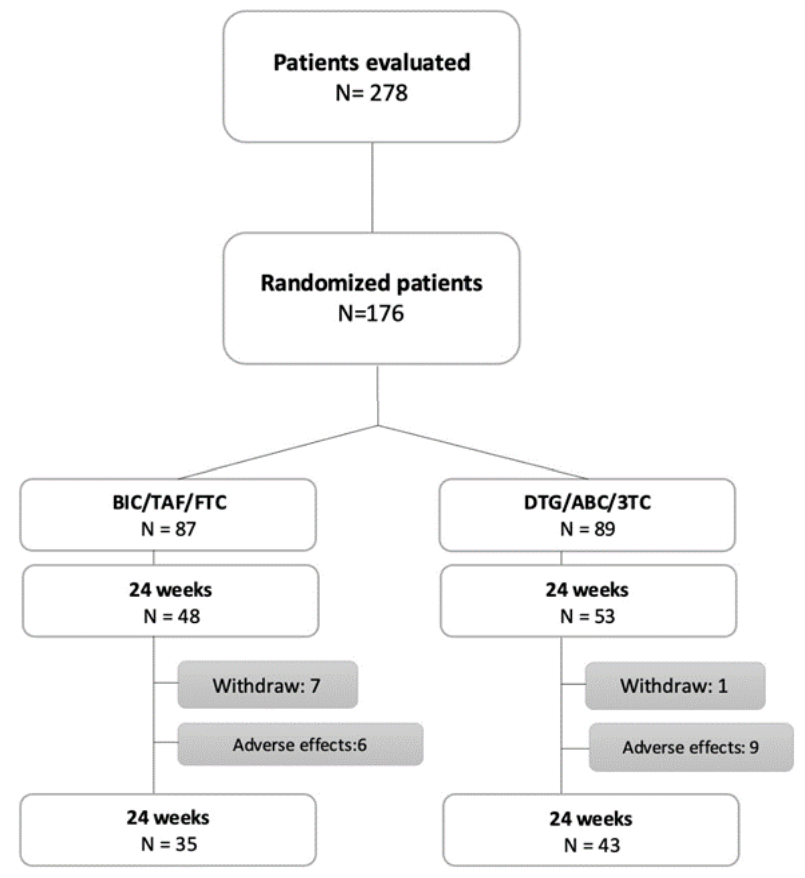
Flowchart diagram describing the inclusion of participants.
The median age for the BIC/TAF/FTC group was 26 years (IQR 23-30), median CD4+ cells count 319 cells/mm3 (IQR 197 -390) and median HIV-1 RNA 4.5 log (IQR 3.9 – 4.7). For the DTG/ABC/3TC group, the median age was 26 years (IQR 22-31) with a median CD4+ cells count of 283 cells/mm3 (IQR 220-360), and median HIV-1 RNA 4.5 (3.7 – 4.9). Baseline characteristics are shown in Table 1.
Baseline characteristics of study population
In the DTG/ABC/3TC group, 43 participants reached week 24 of treatment, and a statistically significant weight gain of + 1.6 kg (0.8 – 4.5; p=0.006) to week 24 was observed (Figure 2); in addition, a significant increase in glucose (p=0.008), HDL cholesterol (p= <0.001) and LDL cholesterol (p= 0.031) was found (Table 2).
In the BIC/TAF/FTC group, 35 patients reached 24 weeks of treatment. There was observed a statistically significant weight gain of + 2.4 kg (0.2-4.9; p=0.003) to week 24 (Figure 2); a significant increase in glucose levels (p=0.050), subcutaneous adipose tissue (p=0.032), visceral adipose tissue (p=0.025) was also found. Regarding the increase in BMI, triglycerides and total cholesterol, no statistically significant increase was found (Table 3).
At 24 weeks, an incidence of 2 (5.7%) and 3 (6.9%) cases of metabolic syndrome was reported in BIC/TAF/FTC and DTG/ ABC/3TC groups respectively. No significant difference was found between the incidence of metabolic syndrome, incidence of hypertriglyceridemia and weight gain when both groups were compared (Table 4).
Measurements in DTG/3TC/ABC group from baseline to 24 weeks
Regarding the adverse events, from the patients evaluated, 67 (72%) reported at least one symptom, 29 (31%) in BIC/ TAF/FTC and 38 (40%) in DTG/ABC/3TC (Table 5). Fifteen (16.6%) participants presented neuropsychiatric adverse events grade 3, leading to ART discontinuation in the first 3 months of therapy. They were switched to a non-INSTI based regimen,
with symptoms resolution.
Discussion
The data from this Mexican clinical trial shows in a preliminary analysis that second generation INSTI-based regimens were effective and well tolerated in our population. The incidence of MetS was similar in both groups. In addition, metabolic disturbances such as glucose increase, hypertriglyceridemia, hypercholesterolemia, weight gain, hypertension and gaining
subcutaneous and visceral fat were also similar
Measurements in BIC/FTC/TAF group from baseline to 24 weeks
Similar to our findings, prior studies have suggested that INSTI-based regimens are associated with weight gain. In a pooled analysis of 8 randomized clinical trials of ART naïve PLHIV,
participants taking INSTIs experienced the most weight gain. Bictegravir (BIC) and Dolutegravir (DTG) demonstrated similar weight gain with 4.24 kg and 4.07 kg through week 96 respectively. In our study, the group of BIC/TAF/FTC experienced a weight gain of 2.4 kg and DTG/ABC/3TC group 1.6 kg in the first 24 weeks. Among NRTI analysis, TAF was associated with the most weight gain, similar to our findings where BIC/TAF/FTC experienced greater weight gain9.
Both regimens were well tolerated, with similar discontinuations observed, similar efficacy and side-effects. Increase in glucose associated with second generation INSTI regimens has been reported10; these regimens could be concerned for patients about weight gain, or who have substantial weight gain or metabolic complications once started a second generation INSTI-containing regimen. However, no data are available yet to suggest that such side-effects would be reversed on switching.
Comparison between groups of 24 weeks results
Adverse effects reported to 24 weeks
In the ADVANCE trial, there was significantly more weight gain in DTG-containing regimens, especially in combination with TAF, than with the standard care regimen. The reported incidence of metabolic syndrome at 96 weeks in men on the DTG TAF/FTC group was 4.6%. In our study, at 24 weeks the incidence of metabolic syndrome was 5.7% and 6.9% in BIC/TAF/FTC and DTG/ABC/3TC groups respectively. Despite the fact that it is a small sample of patients, in Mexico the diet of the population is high in calories, so at 96 weeks a higher incidence
of MetS could be expected than that reported in other trials11.
Neuropsychiatric adverse events grade 3 that led to discontinuation met more than 16% in incidence when patients were evaluated with HADS, ISI, PHQ-9 and PSQI. Insomnia, anxiety, suicidality and depression were similar between both groups. Rash associate to DTG/3TC/ABC group lead also to discontinuation.
Limitations of the study include the open-label design. Genotyping was not done before the initiation of antiretroviral therapy. Loss of social security because of loss of an established job
was common in some of our patients. For the moment this preliminary analysis is with a small sample size to find differences between groups. Our patients were only men and most of them were young and increasing weight and metabolic disturbances are not so common in this group of age. Some strengths include that it is the first clinical trial that evaluated MetS and metabolic disturbances in PLHIV in Latin-America; in addition, we were able to measure body composition with bioimpedance, subcutaneous and visceral fat.
A longer follow-up time and number of patients are required to obtain significant results. This trial probably had too few participants to detect differences between the regimens in any metabolic parameter. Participants in this study will continue to be followed up until week 144.
In summary, as was observed in this trial, the incidence of MetS and metabolic disturbances is similar with BIC/FTC/TAF or DTG/3TC/ABC. Regarding other adverse events, the rate of discontinuation was similar between groups. Both regimens were effective and well tolerated.
References
- Bourgi, K., Rebeiro, P. F., Turner, et al. Greater Weight Gain in Treatment-naïve Persons Starting Dolutegravir-based Antiretroviral Therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America, 2020; 70(7), 1267–1274.
- Chow, W., Donga, P., Côté-Sergent, A., et al. An assessment of weight change associated withthe initiation of a protease or integrase strand transfer inhibitor in patients with human immunodeficiency virus. Current medical research and opinion, 2020; 36(8), 1313–1323
- Venter, W., Moorhouse, M., Sokhela, S., et al. Dolutegra vir plus Two Different Prodrugs of Tenofovir to Treat HIV. NEJM, 2019; 381(9), 803–815.
- Kileel EM, Lo J, Malvestutto C, et al. Assessment of Obesity and Cardiometabolic Status by Integrase Inhibitor Use in REPRIEVE: A Propensity-Weighted Analysis of a Multinational Primary Cardiovascular Prevention Cohort of People With Human Immunodeficiency Virus. Open Forum Infect Dis. 2021;8(12):ofab537. Published 2021 Nov 20. doi:10.1093/ofid/ofab537
- Bakal, D. R., Coelho, L. E., Luz, P. M., et al. Obesity following ART initiation is common and influenced by both traditional and HIV-/ART-specific risk factors. The Journal of antimicrobial chemotherapy, 2018; 73(8), 2177–2185
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education
Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol in Adults (Adult
Treatment Panel III). JAMA, 2001; 285(19), 2486–2497.
7. Dobrowolski P, Prejbisz A, Kuryłowicz A, et al. Metabolic syndrome – a new definition and management guidelines: A joint position paper by the Polish Society of Hypertension,
Polish Society for the Treatment of Obesity, Polish Lipid Association, Polish Association for Study of Liver, Polish Society of Family Medicine, Polish Society of Lifestyle Medicine, Division of Prevention and Epidemiology Polish Cardiac Society, “Club 30” Polish Cardiac Society, and Division of Metabolic and Bariatric Surgery Society of Polish Surgeons. Arch Med Sci. 2022;18(5):1133-1156. Published 2022 Aug 30. doi:10.5114/aoms/152921.
8. Cerrato, E., Calcagno, A., Biondi-Zoccai, G., et al. Cardiovascular disease in HIV patients: from bench to bedside and backwards. Open heart, 2015; 2(1), e000174.
9. Sax PE, Erlandson KM, Lake JE, et al. Weight Gain Following Initiation of Antiretroviral Therapy: Risk Factors in Randomized Comparative Clinical Trials. Clin Infect Dis. 2020;71(6):1379-1389
10. Lamorde M, Atwiine M, Owarwo NC, et al. Dolutegravir-associated hyperglycaemia in patients with HIV. Lancet HIV. 2020;7(7):e461-e462. doi:10.1016/S2352- 3018(20)30042-4
11. Venter WDF, Sokhela S, Simmons B, et al. Dolutegravir with emtricitabine and tenofovir alafenamide or tenofovir disoproxil fumarate versus efavirenz, emtricitabine, and tenofovir disoproxil fumarate for initial treatment of HIV-1 infection (ADVANCE): week 96 results from a randomised, phase 3, non-inferiority trial. Lancet HIV. 2020;7(10):e666-e676.
CC BY-SA © El autor(s). 2022. Acceso Abierto. Esta obra está bajo una Licencia Creative Commons Atribución-CompartirIgual 4.0 Internacional, que permiten el uso sin restricciones, distribución y reproducción en cualquier medio o formato, siempre que se le dé la atribución al creador. Si remezcla, adapta o construye sobre el material, debe licenciar el material modificado bajo términos idénticos.